The auroral lights appear as time-varying bands and filaments of light, often of many different colours, often moving about the sky, often showing structures oriented in the vertical direction (qualitatively consistent with the orientation of magnetic field lines), and showing structures that are discrete (such as auroral arcs). The auroral lights do not always move, however, but sometimes are very steady and slowly varying. Green and red displays are particularly common. Examples of the auroral lights can be found in Figures 15.6 and 15.11, as well as in Cravens' [1997] book, the paper of Carlson and Egeland [1995], and the web sites given to you at the end of Lecture 13.
Auroral emissions are produced as a result of energetic electrons and protons from the solar wind
and Earth's magnetosphere colliding with the constituents of Earth's upper atmosphere and ionosphere.
Typically, the
emission process involves multiple steps: first, an atmospheric atom, molecule, or ion X is placed in an
``excited'' electronic state ![]() by a collision with a precipitating electron or ion,
by a collision with a precipitating electron or ion,
![]()
in which the electron loses kinetic energy in the collision; second, the entity ![]() either
relaxes to the ground state by emitting a photon
either
relaxes to the ground state by emitting a photon
![]()
relaxes to a lower excited state by emitting a photon, or transfers excess energy to another atmospheric/ionospheric constituent via a collision, etc. Vibrationally excited states are also excited, leading to infrared emission. Note that these de-excitation photons have frequencies and lifetimes that are predicted very accurately by quantum mechanics. The precipitating particle can also create additional plasma particles by impact ionization processes, emit X-rays via bremsstrahlung, undergo chemical recombination reactions, heat the atmosphere and ionosphere etc.
The spectrum of Earth's auroral emissions is composed primarily of many spectral lines and bands in the infrared, the optical band, and the far ultraviolet (Figure 17.1).
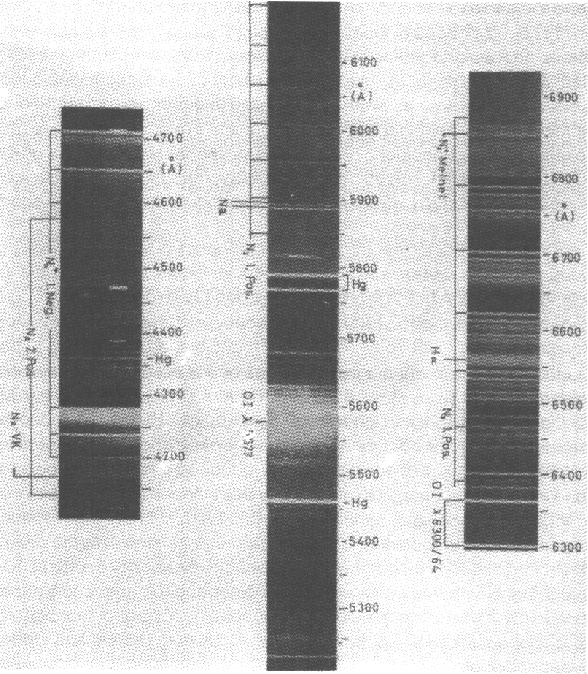
Figure 17.1: Selected portions of auroral spectra in the visible [Vallance Jones, 1974],
showing the discrete nature of the auroral lines.
Bremsstrahlung X-rays are also detected. These spectral lines and bands allow the chemical composition of the atmosphere and ionosphere to be probed remotely, as well as allowing constraints to be placed on the nature, spectrum and characteristic energies of the precipitating particles. For instance, knowing the physics of (say) electron energy loss to certain chemical species and the energy levels of these chemical species, the spectrum, altitude profile, and intensity of radiation can be predicted theoretically and then compared with observations to constrain the electron energy spectrum and/or models for the atmosphere and ionosphere [e.g., Omholt, 1971; Vallance Jones, 1974; Carlson and Egeland, 1995]. These types of calculations are useful for Earth, but also for the recently discovered Jovian auroral displays [Connerney et al., 1996] where detailed comparisons with in situ data are only now becoming possible due to the Galileo spacecraft.
For definiteness, consider the following lines. The brightest visible feature of Earth's aurora is the
so-called ``green line'' at 557.7 nm.
This is due to the transition of an electron from the ![]() S excited state of atomic oxygen to the
S excited state of atomic oxygen to the
![]() D state (Figure 17.2)
D state (Figure 17.2)
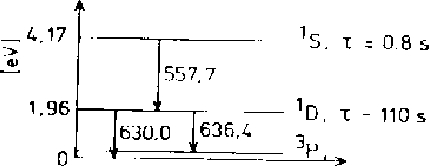
Figure 17.2: Energy level diagram for atomic oxygen [Carlson and Egeland, 1995]. The
radiative half-lives ![]() and wavelengths of the emitted photons are also shown.
and wavelengths of the emitted photons are also shown.
The 630.0 nm line is a ``red line'', produced as the ![]() D state relaxes to the ground state,
that is often observed at high altitudes over the cusp and polar cap (where the atomic
collision time is long compared with the de-excitation time). Note that both these lines
correspond to relatively small excitation energies
D state relaxes to the ground state,
that is often observed at high altitudes over the cusp and polar cap (where the atomic
collision time is long compared with the de-excitation time). Note that both these lines
correspond to relatively small excitation energies ![]() eV, well below the characteristic
energy loss of about 40 eV per ion pair predicted by theoretical calculations [e.g., Carlson and
Egeland, 1971]; accordingly both these lines correspond to collisions by relatively slowly moving primary
particles.
eV, well below the characteristic
energy loss of about 40 eV per ion pair predicted by theoretical calculations [e.g., Carlson and
Egeland, 1971]; accordingly both these lines correspond to collisions by relatively slowly moving primary
particles.
The heights of aurorae are discussed a little more below; however, typically aurorae are formed at heights between 90 and 200 km, thereby corresponding to the thermosphere and ionosphere (Figures 16.1, 16.2, 16.6 and 16.7). Characteristic intensities range from a few tenths to more than 10 kilo-Rayleighs (kR).
The energies of the precipitating electrons and protons are discussed more below. For now, however,
it is enough to state that those originating in the solar wind/magnetosheath have characteristic energies
of order 100 eV, those starting in the plasmasheet have energies of order 1 keV, while those found in
the nightside auroral zone itself have energies in the range of ![]() keV.
keV.